ISO 24495-1 – the first standard for Plain Language

The presentation of medical and pharmaceutical topics often does not address lay audiences. This then begs the question: How do you ensure that everyone can access such important content? The answer involves the use of plain language and the application of ISO 24495-1. This blog post will take a closer look at the concept of plain language, explain why it is so important and present the approaches adopted in ISO 24495-1.
What is plain language and why is it important?
Texts written in plain language put the reader first. In terms of language style, structure and layout, these texts are designed to ensure that readers
- find the information they need,
- understand that information and
- are able to use that information.
With plain language, various texts and information can be presented in a simplified style to appeal to a wider audience. When organizations such as companies, authorities and NGOs use plain language so readers can better understand and process the content, those readers will also put greater trust in the respective organizations. This not only improves the relationship between the organization and intended target group, but also makes the translation process for texts in plain language more efficient. The clearer and easier to understand a text is worded, the more efficiently it can be translated.
Plain language is used in a wide range of settings, for example, in technical writing, in the creation of legal texts, for patient information in medicine and pharmacy, and in other documents intended for the general public.
Plain Language in Clinical Trials

One of the uses of plain language in medicine and pharmacy is in clinical trials. This allows study documents and results to be presented in a manner understood by all participants. Clinical trials rely on different types of texts to communicate study information in a way that laypersons can understand.
1. Lay summaries
Lay summaries are easy-to-understand summaries of study outcomes that scientists can share with the general public.
2. Lay Protocol Synopses
Lay Protocol Synopses detail the study structure and workflow in plain language.
3. Plain Language Summaries
Plain language summaries, on the other hand, summarize publications on the study outcomes in lay terms.
What is the difference between Plain Language and Easy Language?
In addition to technical language and plain language, there is another category: Easy Language. While the target group for plain language is readers without any particular expertise, easy language texts are aimed at people with difficulties in reading and/or understanding. This avoids foreign words completely, relies on short and concise sentences, and employs visual tools such as pictures and lists. Easy language thus also ensures improved accessibility.
ISO 24495-1 – a brief explanation
In 2023, the International Organization for Standardization, better known as ISO, published a standard for plain language - ISO 24495-1. The ISO sets out principles and guidelines serving as recommendations for creating documents in plain language. The focus here is on printed and digital information, most of which is available as text. However, the guidelines may also be used as a tool when creating comprehensible and simple content for podcasts and videos.
The four governing principles of ISO 24495-1
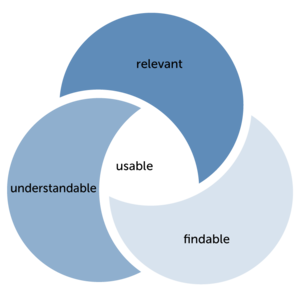
ISO 24495-1 defines four governing principles. These principles are based on the assumption that documents are useful if the information they contain is relevant, easy to find and understandable. These governing principles are implemented through guidelines describing the principles in more detail.
"Principle 1: Readers get what they need (relevant)."1
When creating relevant content, authors should first identify who the readers are and what their objectives and context are when reading the text. This is the basis on which the appropriate document type is selected and the content needed by the readers is created accordingly.
"Principle 2: Readers can easily find what they need (findable)."2
The document should be well structured, for example, by using subheadings, so that readers can find all the information they need. This allows readers to recognize at one glance where in the text they can find which information. If additional information, such as background knowledge, is needed, this should be listed separately.
"Principle 3: Readers can easily understand what they find (understandable)."3
By using words familiar to them, readers can understand the information more easily. In addition, clear and concise sentences and paragraphs can also improve comprehension. Images and multimedia elements can help to convey complex information. Moreover, the writing style and tone of the text should match the situational needs of the reader. And the authors should also ensure that the document as a whole is coherent.
"Principle 4: Readers can easily use the information (usable)."4
For the document to be useful for the reader, one thing is paramount: continuous review. The document should be constantly scrutinized for the above aspects during its creation. After completion and before publication, the readers from the target group should evaluate the document. But even after publication and use of the document, it should continue to be reviewed whether it complies with the governing principles and adapted, if necessary.
Clinical Trials and Medical Writing at mt-g

Implementing the complex governing principles and guidelines of the new ISO standard can be a challenge taking up time and resources. Our Clinical Trials team is at your side as a valuable partner. Our experienced medical writers are not only well acquainted with the latest developments in your specialty, but are also proficient in the practical application of ISO 24495-1.
However, our services extend way beyond medical writing. We also offer expert translations in medicine and pharmacology, back translations, adaptations, and reviews for each phase of your clinical trial. One of our strong points is on-time delivery so that your study phases can be completed efficiently and precisely. Even last-minute changes are no problem for us – we implement them quickly and reliably.
Our dedicated team will provide you with professional support that will take your projects to a new level and save you time and resources. Please contact us to find out more about how we can help you with your clinical trials.


