Your one-stop service provider
Our complete service package for your EU regulatory labelling needs
Are you looking for a language service provider for your regulatory labelling needs that …
- assists you even with creating your source texts,
- offers you expert support within the context of the regulatory process,
- handles all communications with the authorities,
- is familiar with the tight regulatory timelines and requirements of the respective national authorities,
- implements changes flexibly, even at short notice, and
- translates your product information into all required languages?
Then our comprehensive regulatory labelling service is just what you need.
From application to authorization
We take care of all regulatory labelling matters.
The product information is the central document in any authorization dossier. It is subject to a multitude of regulatory requirements, and is one of the few documents publicly accessible. It therefore forms an important part of how your product is perceived externally. In the centralized EU authorization procedure, the texts must also be made available in all 26 EU/EEA languages.
Our expertise for your project
Together with our long-term partner in the field of regulatory labelling, we will guide you through all of the process steps. We will help you to create your source texts, produce professional specialized translations of your product information, and communicate with the competent authorities on your behalf.
Our complete service package for regulatory labelling includes:
- Strategic regulatory advice across the entire product cycle within the context of European authorization procedures (e.g., new MAA, variations, renewals, line extensions)
- Support in the creation and optimization of the English text (e.g., ensuring consistency with CCDS/CCSI, possible combinations of product strengths, optimization of package leaflet readability)
- Professional translations of your product information and revision by our regulatory affairs specialists and specialized native-speaker translators in all required languages
Timely management of the entire regulatory process between day 0 and day 235/day +25, including:
- Coordination of all activities related to the creation of the language versions
- Coordination of the verification of all language versions by the customer’s national representatives, on request
- Coordination of all interactions with the 26 national competent authorities (NCAs) as part of the linguistic review process, including Eudralink submissions, if requested
- Lifecycle management of your product information texts
A flawlessly designed service from beginning to end
in the field of regulatory labelling
- You can rely on us.
Your project manager is your personal point of contact. They will provide you with expert advice and guide you through the entire labelling process. - mt-g's complete service package
We combine our regulatory labelling expertise with our many years of experience in the professional translation of labelling documents. - Our reliable time management
We know how important it is to comply with deadlines during the authorization process of your medicinal products. That is why we always monitor all timelines for the timely submission of your documents. - Outstanding process expertise
Our project managers are deeply familiar with all requirements of the national and European regulatory authorities. - Two reliable partners committed to your success
We have been conducting regulatory labelling projects together with a partner company for many years. Together, we can draw on more than 60 years of expertise. We will complete your project to the highest standards.
The final 300 days in a centralized authorization procedure
and the all-in-one solution for your product information
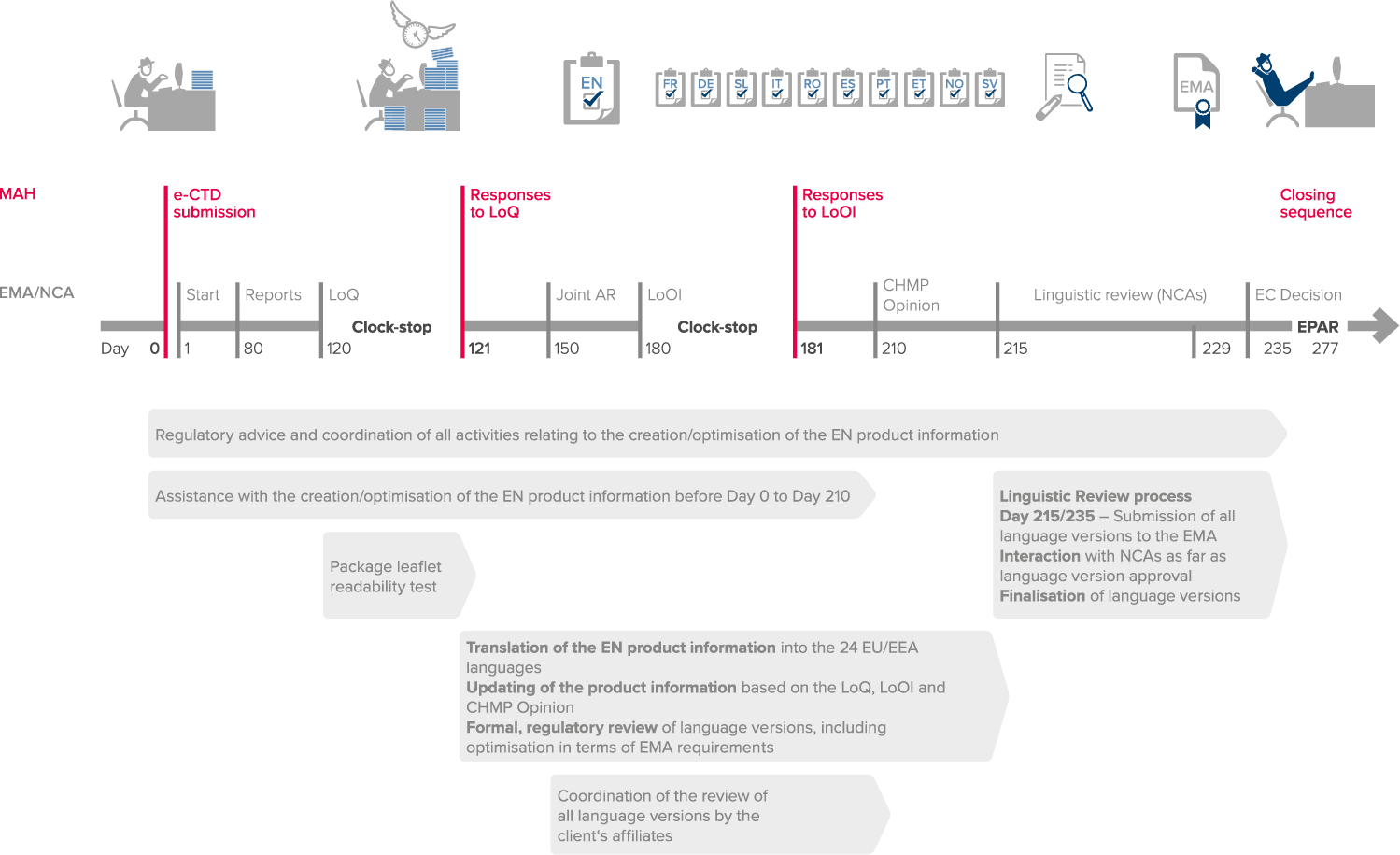
The final 300 days in a centralized authorization procedure
and the all-in-one solution for your product information


